Introduction
l. Plain carbon steel owes its properties to the presence of carbon. Steels which owe their properties to elements other than carbon are termed alloy steels. The effects of carbon when added to iron are best understood by first considering pure iron.Pure iron is very ductile, and has a tensile strength of 18 tons per sq. in. The addition of carbon to the iron increases the tensile strength, hardness and brittleness. and at the same time decreases the ductility (0.87 per cent. carbon steel has a tensile strength of approximately 55 tons per sq. in.).
The increase in strength of steel with increase in carbon content is limited to the amount of carbon which will remain combined with the iron after any normal heat treatment.
Above 1.7 per cent. carbon, the excess carbon is in free state or graphite, which has Very low strength. The carbon content of plain carbon steel rarely exceeds 1.5 per cent.
2. When steel is heated, the internal structure of the steel changes and this affects its properties. These changes, in the reverse order, also take place on cooling.
Prolonged heating or working of the steel produces some other effects; therefore, a knowledge of the heat treatment processes is a necessity. For example, by using the correct heat treatment, medium carbon steel may be made soft, hard or brittle, depending on the purpose for which steel is to be used.
Heat Treatment of Carbon Steel
3. The internal structure of steel may be varied by heating and cooling. To produce certain properties in the steel, it is subjected to processes termed heat treatment.
It is not always possible to produce all the desired properties in the metal and a compromise has to be made. Considerations of easy manufacture may necessitate softness of the metal, while requirements of use may demand great strength and considerable hardness.
Thus, if a steel is required to be hard l and tough, the maximum degree of hardness and toughness cannot be obtained together, since maximum hardness promotes brittleness and maximum toughness generally reduces hardness.
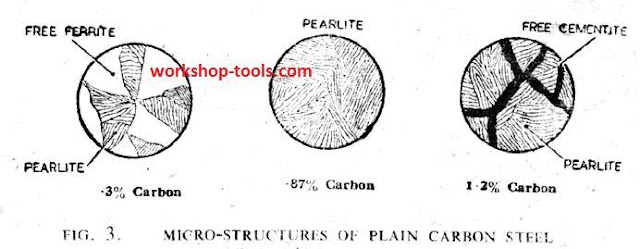
4. Structure of Steel. The constituents of at carbon steel are pure iron (ferrite), and a chemical compound of carbon in iron termed iron carbide (cementite). In a low carbon steel, these constituents when viewed through a microscope appear as a laminated structure (pearlite), surrounded by free ferrite.
With increasing carbon content, the proportion of pearlite to ferrite increases until,with a 0.87 per cent. carbon content, the steel becomes wholly pearlitic.
Above 0.87 per cent. carbon content, a microscopic examination reveals pearlite surrounded by free cementite. Ferrite is soft, ductile, and not very strong pearlite is strong and tough, but soft enough to be readily worked; ccmentite is very rand and brittle.
Thus, as the carbon content is increased up to 0.87 per cent., the steel gets tougher and stronger, but beyond this percentage, due to the increasing cementite content, the steel becomes very hard and progressively brittle.
5. Critical Points. When steel is heated. its temperature does not increase at a constant rate. The temperature rise, after proceeding steadily, is momentarily checked and after a short interval. the temperature continues to rise as before.
During this period of arrest the metal absorbs heat.but instead of raising temperature. the heat brings about certain changes in the metal. The temperatures at which these periods occur are termed “critical” or “arrest” points.
6. ln the equilibrium diagram. the critical points are shown on a rising temperature, The line AEB represents the lower critical points, and the line DEC the upper critical points. It will be seen that although the steels vary in carbon content they all have the same lower critical point (730°C).
At this temperature, the pearlite as such disappears and the laminae of Ferrite and eementite, of which it is composed, dissolves and forms a solid solution known as austenite which is non-magnetic.
The ferrite and cementite of pearlite are in such proportion that the mixture forms a solid solution at a lower temperature than when they are mixed in any other proportion. i. e., pearlite is the eutectoid of steel.
As 0.87 per cent. carbon steel consists entirely of pearlite, it has only one critical point, therefore, steels containing a lower or higher carbon content than 0.87 per cent. must be subjected to increase in temperature (upper critical point) to bring the free ferrite or cementite into solid solution. When steel is allowed to cool slowly these changes occur in the reverse order, but at a temperature some 30°C lower.

Comments
Post a Comment