Critical bodies such as aircraft structures are designed to provide maximum strength with minimum weight therefore unchecked corrosive attack would have serious consequences resulting in the failure of the structure.
The following methods are employed to prevent corrosion.
(a) By making the metal to form its own protective coating for example, anodizing aluminium and its alloys, chrornating magnesium and cosleitising steel.
(b) Covering the surface with another metal less likely to corrode for example, galvanizing steel, hot-rolling aluminium (alclad), and metal spraying.
(c) Covering the surface with a protective agent for example, enamel, paint, varnish, oil, grease or lanolin, depending on the material to be protected.
Anti-corrosive treatment is given during manufacture to most parts of an airframe as a protection against corrosion and deterioration. The treatment of parts normally provides a very thin anti-corrosive film which is generally covered by a protective finish to give added protection and improve its anti-corrosive qualities.
If the anti-corrosive film is scratched or broken, not only does the exposed metal corrode, but the corrosion may also spread under the surrounding film. Every care must therefore be taken, to prevent surfaces from being scratched or damaged.
Where damage o the anti-corrosive treatment exists and it is impracticable to have the whole part removed for treatment, the best possible substitute should be used which would consist of, applying protective finish, oil or grease, depending on the part to be treated.
When repairing damaged parts, corroded areas must be regarded as damaged areas and included in the damage classification. Great care must be taken to ensure that no corrosion remains undetected, especially where it will not be seen.
For example, surface corrosion may not be visible to the naked eye when the metal is covered by protective finish, but it may be detected by pressing the protective finish with the finger when the paint or enamel will flake off. The extent of corrosion of rivet heads can be determined by rubbing the heads with a piece of hardwood.
The parts and the plating metal are suspended separately from brass or copper bars fitted across the top of the tank.
The bars are connected to a direct cur»rent supply; “negative” being connected to the bar supporting the parts and “positive” to the bar Supporting the plating material. When electric current passes through the tank a thin film of metal is deposited on the parts as the plating metal is slowly consumed.
In electro-plating, which is a form of electrolysis, special electrodes are used and metallic salts are added to the electrolyte to ensure that the minimum gas is emitted, while maximum effect is obtained in depositing the metal.
In principle the current causes‘ metal from the electrolyte to be deposited on to the cathode, while the anode slowly dissolves to maintain the metal content of the electrolyte.
The equipment and process used for various kinds of electroplating such as cadmium plating, Nickel plating,
Chromium plating and Zinc plating are more or less the same except for the electrolyte, plating metal, current density, voltage, temperature of the bath and duration of the process.
The most commonly used plating metals are cadmium, chromium, nickel, zinc, copper and tin. For example, A.G.S. parts are cadmium or zinc plated; and cockpit or cabin fittings etc., are nickel, copper or tin plated.
To provide a hard-wearing surface and anti-corrosive treatment, oleo leg sliding members and ball bearings are usually chromium plated. To obtain greater corrosion resistance, one plating metal may be plated on to another e.g., chromium on nickel.
In this instance, splitting of the electrolyte into hydrogen and oxygen is the important factor, the hydrogen forming at the cathode and the oxygen at the anode.
The oxygen combines with the anode, which is the part being-treated, to form a continuous surface film consisting mainly of hydrated aluminium oxide, which has a high resistance to corrosion.
Chromic acid or sulphuric acid is used as the electrolyte.
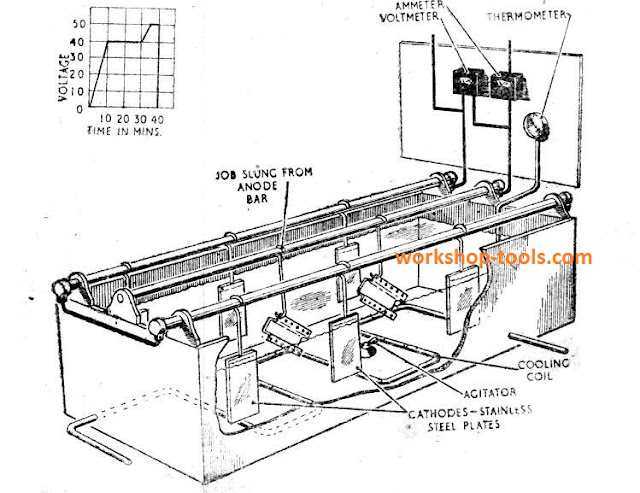
The tank itself may be lined with stainless steel plates and serve as the cathode, although stainless-steel plates suspended from a cathode bar are sometimes used.
The bath is worked at a temperature of 40 °C +/- 2°C., and the electrolyte must be gently agitated; a rotating paddle is a commonly used method. A thermometer is used to check the temperature, which can be regulated by operating a cooling/heating system consisting of a cold or hot water coil fitted along the length of the bath.
The direct current supply used to operate the bath ranges from 0 to 50 volts.
(a) Process: Before treatment, the parts are cleaned by a degreasing agent to remove dirt, oil or grease, then washed in clean running water. After cleaning, they must not be touched with the bare hands and are suspended by aluminium wire from the anode bar and immersed in the electrolyte.
With the bath at the correct temperature, and the agitator operating, the current is switched on and controlled as follows:
(i) FIRST 10 MINUTES. Voltage across the bath gradually increased. in steps
of not more than 5 volts, from 0 to 40 volts.
(ii) NEXT 20 MINUTES. Voltage maintained steady at 40 volts.
gin) NEXT 5 MlNUTES. Voltage increased‘ gradually to 50 volts.
(iv) NEXT 5 MINUTES. Voltage maintained at 50 volts.
(v) SWITCH OFF CURRENT.
(Total time required :4-0 minutes).
Immediately after treatment, the parts are swilled in clean running water followed by washing in hot water and drying.
The quality of the film can be determined by appearance, feel, dye, and electrical tests. The dye test consists of marking the parts with an indelible pencil or cheap dye; if the film is satisfactory, the dye cannot be removed with a damp cloth.
Although the wire is melted at high temperature, the speed “of travel effectively cools the sprayed metal and does not cause heating of the’treated part Aluminium, which is difficult to deposit by other methods, can be sprayed.
When aluminium is used. the process is termed Aluminizing Magnesium alloy nose or tail wheel hubs are sometimes aluminized.
The following methods are employed to prevent corrosion.
(a) By making the metal to form its own protective coating for example, anodizing aluminium and its alloys, chrornating magnesium and cosleitising steel.
(b) Covering the surface with another metal less likely to corrode for example, galvanizing steel, hot-rolling aluminium (alclad), and metal spraying.
(c) Covering the surface with a protective agent for example, enamel, paint, varnish, oil, grease or lanolin, depending on the material to be protected.
Anti-corrosive treatment is given during manufacture to most parts of an airframe as a protection against corrosion and deterioration. The treatment of parts normally provides a very thin anti-corrosive film which is generally covered by a protective finish to give added protection and improve its anti-corrosive qualities.
If the anti-corrosive film is scratched or broken, not only does the exposed metal corrode, but the corrosion may also spread under the surrounding film. Every care must therefore be taken, to prevent surfaces from being scratched or damaged.
Where damage o the anti-corrosive treatment exists and it is impracticable to have the whole part removed for treatment, the best possible substitute should be used which would consist of, applying protective finish, oil or grease, depending on the part to be treated.
When repairing damaged parts, corroded areas must be regarded as damaged areas and included in the damage classification. Great care must be taken to ensure that no corrosion remains undetected, especially where it will not be seen.
For example, surface corrosion may not be visible to the naked eye when the metal is covered by protective finish, but it may be detected by pressing the protective finish with the finger when the paint or enamel will flake off. The extent of corrosion of rivet heads can be determined by rubbing the heads with a piece of hardwood.
Electro-Plating
Steels, brasses and bronzes may be electro-plated. The process consist of suspending the parts to be plated, together with the plating metal, in a tank containing a chemical solution termed an electrolyte.The parts and the plating metal are suspended separately from brass or copper bars fitted across the top of the tank.
The bars are connected to a direct cur»rent supply; “negative” being connected to the bar supporting the parts and “positive” to the bar Supporting the plating material. When electric current passes through the tank a thin film of metal is deposited on the parts as the plating metal is slowly consumed.
In electro-plating, which is a form of electrolysis, special electrodes are used and metallic salts are added to the electrolyte to ensure that the minimum gas is emitted, while maximum effect is obtained in depositing the metal.
In principle the current causes‘ metal from the electrolyte to be deposited on to the cathode, while the anode slowly dissolves to maintain the metal content of the electrolyte.
The equipment and process used for various kinds of electroplating such as cadmium plating, Nickel plating,
Chromium plating and Zinc plating are more or less the same except for the electrolyte, plating metal, current density, voltage, temperature of the bath and duration of the process.
The most commonly used plating metals are cadmium, chromium, nickel, zinc, copper and tin. For example, A.G.S. parts are cadmium or zinc plated; and cockpit or cabin fittings etc., are nickel, copper or tin plated.
To provide a hard-wearing surface and anti-corrosive treatment, oleo leg sliding members and ball bearings are usually chromium plated. To obtain greater corrosion resistance, one plating metal may be plated on to another e.g., chromium on nickel.
Anodizing
This process is applied to aluminium and aluminium alloys, and is similar in principle to electro-plating, but the current is passed between two electrodes, neither of which dissolves.In this instance, splitting of the electrolyte into hydrogen and oxygen is the important factor, the hydrogen forming at the cathode and the oxygen at the anode.
The oxygen combines with the anode, which is the part being-treated, to form a continuous surface film consisting mainly of hydrated aluminium oxide, which has a high resistance to corrosion.
Chromic acid or sulphuric acid is used as the electrolyte.
Chromic Acid Method.
The electrolyte consists of 3‘ percent. (by weight) of chromic acid in distilled water. Brass anode bars are fitted across the top of the tank and from these the parts to be treated are suspended and immersed in the electrolyte.The tank itself may be lined with stainless steel plates and serve as the cathode, although stainless-steel plates suspended from a cathode bar are sometimes used.
The bath is worked at a temperature of 40 °C +/- 2°C., and the electrolyte must be gently agitated; a rotating paddle is a commonly used method. A thermometer is used to check the temperature, which can be regulated by operating a cooling/heating system consisting of a cold or hot water coil fitted along the length of the bath.
The direct current supply used to operate the bath ranges from 0 to 50 volts.
(a) Process: Before treatment, the parts are cleaned by a degreasing agent to remove dirt, oil or grease, then washed in clean running water. After cleaning, they must not be touched with the bare hands and are suspended by aluminium wire from the anode bar and immersed in the electrolyte.
With the bath at the correct temperature, and the agitator operating, the current is switched on and controlled as follows:
(i) FIRST 10 MINUTES. Voltage across the bath gradually increased. in steps
of not more than 5 volts, from 0 to 40 volts.
(ii) NEXT 20 MINUTES. Voltage maintained steady at 40 volts.
gin) NEXT 5 MlNUTES. Voltage increased‘ gradually to 50 volts.
(iv) NEXT 5 MINUTES. Voltage maintained at 50 volts.
(v) SWITCH OFF CURRENT.
(Total time required :4-0 minutes).
Immediately after treatment, the parts are swilled in clean running water followed by washing in hot water and drying.
The quality of the film can be determined by appearance, feel, dye, and electrical tests. The dye test consists of marking the parts with an indelible pencil or cheap dye; if the film is satisfactory, the dye cannot be removed with a damp cloth.
Sulfuric Acid Method
The equipment is similar to that used for the chromic acid method, but the tank is lead-lined, the cathodes are of lead instead of stainless steel, and the direct current supply does not exceed 14 volts. The eletrolyte consists of 18 to 22 %. (by volume) of sulfuric acid in distilled water.Metal Spraying
Metals or alloys capable of being drawn into wire can be melted and sprayed on to previously prepared surfaces of metals with an oxy-hydrogen pistol.Although the wire is melted at high temperature, the speed “of travel effectively cools the sprayed metal and does not cause heating of the’treated part Aluminium, which is difficult to deposit by other methods, can be sprayed.
When aluminium is used. the process is termed Aluminizing Magnesium alloy nose or tail wheel hubs are sometimes aluminized.
Comments
Post a Comment