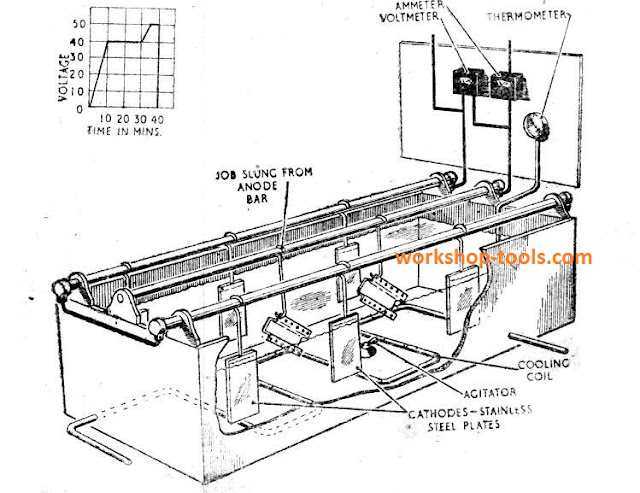
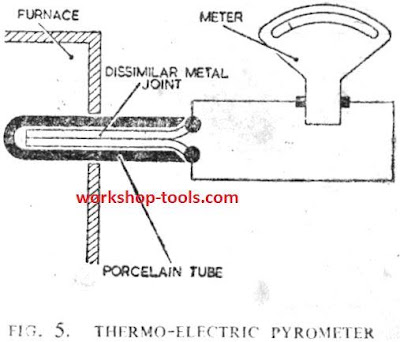
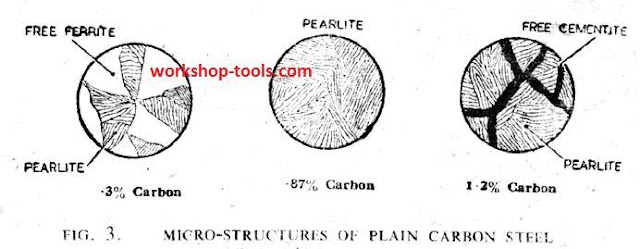
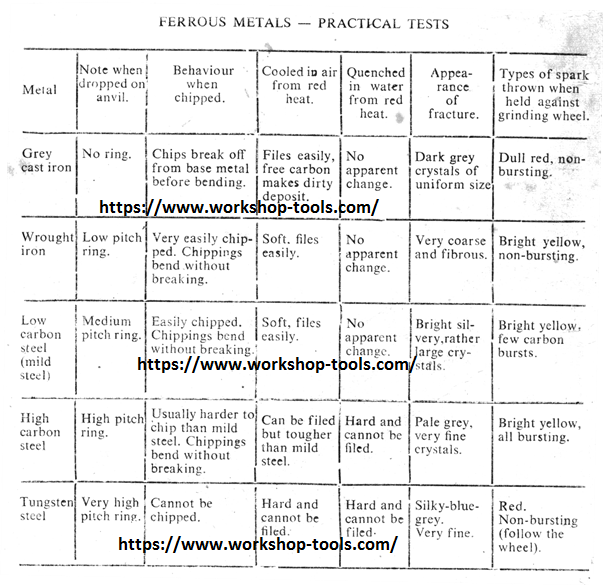
Heat Treatment of Non-Ferrous Metals and Alloys
Method of Heating Metals such as Duralumin and aluminum are heat treated in a salt bath. This is a mild steel bath containing a mixture of potassium an sodium nitrates, which is heated by gas, oil and electricity. Heat Treatment of Duralumin Anealing: This operation permits the material to be worked and bent. Duralumin is annealed in a salt bath at a temperature between 360 degrees Celsius and 400 degrees Celsius and then quenched in clean cold water. Normalizing: Duralumin is normalized to relieve stresses and strains set up by working. Duralumin is normalized in a salt bath at a temperature between 485 degrees Celsius, and 505 degrees Celsius an then quenched in clean water. Note: Normalized Duralumin gradually becomes harder and stronger (age-hardens) during the three or four days after treatment. Heat Treatment of Aluminium Annealing is the only heat treatment applied to aluminum. Sheet which has been hardened by cold working can be restored to a soft ductile condition